Table of Content
Requirements of Food for Special Dietary Use (FSDU) Category As per FSSAI (Nutra) Direction, 2022

India took part in the 44th Codex Committee on Nutrition and Foods for Special Dietary Uses (CCNFSDU) session, which took place from October 2, 2024, to October 6, 2024, in Dresden, Germany. India made crucial interventions on important agenda issues as a major contributor.
It extended its support to create harmonised probiotic guidelines for dietary supplements and offered insightful information on nutrient reference values for children between the ages of six and thirty-six months. The Codex Alimentarius Commission (CAC) section CCNFSDU oversees creating international standards for special diet foods such baby formulae, nutritional supplements, and medical foods.
With input from its 189 Codex Members (including India), the CAC was founded in 1963 by the Food and Agriculture Organisation (FAO) and the World Health Organisation (WHO). Its goals are to protect consumer health and encourage fair trade practices.
India During the 44th CCNFSDU Session
India emphasised that the FAO/WHO probiotic guidelines from 2001 and 2002 need to be updated, as they are 20 years old. The nation demanded unified rules to improve international trade. The CCNFSDU approved India’s recommendation that the combined Nutrient Reference Value-Requirement (NRV-R) for children 6 to 36 months be determined by averaging the two age groups (6–12 months and 12-36 months).
NRVs-R are dietary recommendations for target groups or populations that are based on the most recent scientific research.
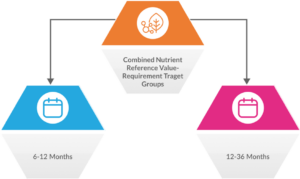
Fig. 1: Combined Nutrient Reference Value-Requirement (NRV-R) for Children
Definition of Food for Special Dietary Use
Food for Special Dietary Use is food prepared or formulated specifically to meet the dietary needs of people with particular health conditions or physiological conditions. Such conditions comprise:
- Low weight
- Obesity
- Diabetes
- High blood pressure
- Pregnancy and lactation
FSDU is designed for dietary management and should not be provided for people two years of age or older.
Categories covered under the regulations – Food Safety and Standards (Health Supplements, Nutraceuticals, Food for Special Dietary Use, Food for Special Medical Purpose, and Prebiotic and Probiotic Food) Regulations, 2022 – include the following:
- Health Supplements (HS)
- Nutraceuticals (Nutra)
- Food for Special Dietary Use (FSDU)
- Food for Special Medical Purpose (FSMP)
- Prebiotic food and Probiotic food (Pre-Pro)
Scope of Products
FSDU includes products formulated for individuals who may:
- Have specific physiological or metabolic disorders/conditions.
- Be undergoing medical supervision for a specific health condition.
- Require a controlled intake of certain nutrients (e.g., low-protein diets for individuals with kidney disorders) .
FSDU does not include products that are meant to treat, cure, or prevent any disease.
Nutritional Composition
FSDU must contain ingredients that are appropriate for the target group and adhere to the following:
- The nutrient content should align with the purpose for which the product is intended.
- The product must contain energy, protein, fats, vitamins, and minerals as per the standards laid down by FSSAI. The specific nutrient composition should be clearly outlined based on its use.
- Nutrient values should not exceed Recommended Daily Allowances (RDA) unless there is a scientific rationale provided.
- If designed for individuals with specific health conditions (e.g., low-sodium, gluten-free), it must comply with the particular dietary requirements mentioned.
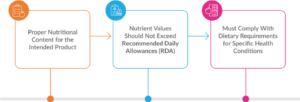
Fig. 2: Nutritional Requirements for Food for Special Dietary Use (FSDU)
Labelling Requirements
FSDU products must have clear and accurate labelling to ensure that consumers can make informed decisions. The labelling must include:
- A clear statement identifying the product as “Food for Special Dietary Use”.
- The target group for the product (e.g., “For individuals with diabetes” or “For individuals with lactose intolerance”).
- The phrase “Not for medicinal use” should be prominently displayed.
- Specific instructions for use, including preparation, administration, and dosage recommendations.
- Storage conditions and shelf-life should be clearly mentioned.
- If the product is designed for use under medical supervision, it should include the statement: “Use under medical or dietetic supervision”.
Safety and Quality Standards
- All FSDU products must comply with the general food safety and hygiene standards set by FSSAI.
- The products must not contain any substances harmful to the intended group, and manufacturers must ensure that all ingredients and additives used are safe for the specific population.
Prohibitions
- FSDU products must not make unsubstantiated claims regarding the treatment, cure, or prevention of any disease or condition.
- FSDU products should not be promoted as a substitute for a balanced diet for the general population unless specifically recommended for individuals with the stated condition.
Packaging Requirements
The packaging of FSDU products should be designed to protect the contents from contamination and preserve the product’s integrity and nutritional value throughout its shelf life. It should also include:
- Tamper-proof packaging
- Clear information on serving sizes and recommended daily intake
Registration and Approval
- Manufacturers and importers of FSDU must obtain prior approval from FSSAI before introducing products to the market.
- The application for approval should include detailed formulations, safety data, nutritional composition, and any other relevant information.
Scientific Evidence
- Manufacturers must maintain scientific evidence to substantiate the product’s efficacy and its suitability for the specified dietary use.
- Evidence of clinical studies or scientific literature supporting the product’s benefits for the specific target group must be available.
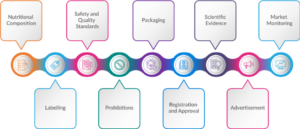
Fig. 3: Key Components of FSDU Regulations
Advertising and Promotion
- FSDU products should not be promoted as a replacement for normal diet except for the individuals they are specifically intended for.
- No misleading claims or exaggerated health benefits should be made in advertising or promotional materials.
Market Monitoring
- FSSAI reserves the right to conduct regular audits and inspections to ensure compliance with the FSDU guidelines.
- Any product found non-compliant with the regulations could face penalties, recalls, or market bans.
Conclusion
The FSSAI (Nutra) Direction 2022 sets strict guidelines for the manufacturing, labelling, and marketing of Food for Special Dietary Use (FSDU) products in India. These regulations ensure that FSDU products cater to the specific dietary needs of individuals with medical conditions while maintaining the highest safety and quality standards. Manufacturers must comply with these guidelines to ensure product safety and efficacy for the target population.
Enquire Now
To enquire about our services please complete the form below and we will be in tough with you as soon as possible
Food Regulatory Services
- Consumer Product
- Compliance Services
- Licenses