Table of Content
Regulatory Requirements of Spices as Per the Food Safety and Standards Authority of India (FSSAI)

Summary
- Food Safety and Standards Authority of India (FSSAI) has standardized spices, condiments and related products under regulation 2.9.
- Microbiological testing is mandatory for these products.
- Proper handling of spices post-harvest should be followed according to FSSAI requirements.
- Powdered spices are to be sold in packets following FSSAI guidelines.
- Maximum Residual Limits (MRLs) for pesticides in spices are specified.
- Spice analysis is to be done using the manual available from FSSAI.
Short Description
The Food Safety and Standards Authority of India (FSSAI) has set regulatory guidelines to monitor the safety of spices, condiments, and related products. This article explores these regulations in detail and provides a comprehensive overview.
FSSAI Standardisation of Spices and Condiments
Spices are an integral part of Indian cuisine and a valuable export commodity. FSSAI regulates the quality of salt, spices, condiments and related products under “2.9: SALT, SPICES, CONDIMENTS AND RELATED PRODUCTS” of Food Safety and Standards (Food Products Standards and Food Additives) Regulation, 2011.
This standardisation covers 42 different categories including common and iodised salt, spices, curry powder, mixed masalas, seasonings etc. The regulations specify that extraneous matter in these products should not exceed 2% of the total weight. Each category specifies quality parameters such as moisture content, volatile oil content, ash content, insect damage content and permissible levels of extraneous matter.
Microbiological Requirements for Spices
To ensure spices are safe for consumption microbiological requirements must be maintained. These standards are outlined in Table 3 of Appendix B of the Food Safety and Standards (Food Products Standards and Food Additives) Regulation, 2011. These include:
- Total aerobic colony count: Must be within specified limits
- Yeast and mould count: Must be within specified limits
- Salmonella: Must be absent
- Enterobacteriaceae, Staphylococcus aureus, Sulphite Reducing Clostridia, and Bacillus cereus: Must be within specified limits
These limits may differ in fresh, dried/dehydrated, ground/powder, extracted, or wet/paste spices.
Guidelines on Food Safety Management System (FSMS) for Spice Processing
Recognizing the critical nature of the post-harvest process, FSSAI has developed a detailed guide as outlined below:
- Handling of raw spices – Processing, transportation, packaging and quality control to maintain product safety and integrity.
- Procedures – Cleaning, maintenance, pest control, and waste management to prevent contamination of spices.
- Storage of spices post-harvest – Location, design, equipment, and utilities to ensure a clean and safe environment for food processing.
- Maintenance of transparency and trust – Accurate labelling, consumer education, and effective complaint handling.
- Regular audits – Accurate documentation and record-keeping to support traceability and regulatory compliance.
- Ensure compliance with food safety standards – Routine employee training, supervision, and continuous education.
Additionally, FSSAI describes the hazards associated with spice processing and adulterants found in spices that are to be avoided.
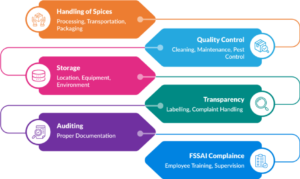
Fig. 1: Food Safety Management Checkpoints for Spice Processing
Restrictions relating to sale conditions of powdered spices
To ensure spices are sold without contamination and quality is maintained, the Food Safety and Standards (Prohibition and Restrictions on Sales) Regulations, 2011, is followed. It states that no one is permitted to sell powdered spices and condiments unless they are sold in packaged form. For regulation 2.3.14 (15), “Spices and Condiments” refer to those specified in section 2.9 of the Food Safety and Standards (Food Products Standards and Food Additives) Regulations, 2011.

Link: https://www.pexels.com/search/spices/
Figure: Different types of powdered spices
Maximum Residue Limits (MRLs) for Pesticides
Recognising the potential hazards of pesticide residues, FSSAI has established maximum residue limits for pesticides in spices and culinary herbs. These limits are specified in the Food Safety and Standards (Contaminants, Toxins and Residues) Regulations, 2011. Key points regarding MRL regulation are as follows:5
- MRLs are based on data from the Central Insecticides Board and Registration Committee (CIB&RC) and Monitoring of Pesticide Residues at National Level (MPRNL) scheme.
- If MRLs are not specified for spices and herbs, Codex MRLs apply. If Codex standards are unavailable, use MRL of 0.1 mg/kg.
- If the pesticide is not registered with CIB&RC, use MRL of 0.1 mg/kg.
- If pesticides are registered and MRLs are specified by these international bodies, their standards apply.
- For unregistered pesticide, use MRL of 0.01 mg/kg (or Below Detection Limit).
The permissible limit of contaminants in spices are listed in Table 1:
| Contaminant | Limit | Unit |
| Arsenic | 5.0 | Parts per Million (mg/kg or mg/L) |
| Lead | 10 | Parts per Million (dry matter basis) |
| Total Aflatoxins | 30 | Micrograms per Kilogram (µg/kg) |
| Aflatoxin B1 | 15 | Micrograms per Kilogram (µg/kg) |
Table 1: Contaminants permissible for food safety in spices
Advanced Analysis Methods for Spices
To ensure compliance with standards, FSSAI provides a comprehensive manual assessing parameters like proximate composition, spice bio-actives, adulteration and volatiles. The manuals provide detailed structure on:
- Sample preparation of spices
- Quality Assessment including the determination of extraneous matter, moisture content, and ash levels in spices and condiments.
- Measurement of volatile and non-volatile components
- Measurement of nutritional components
- Detection of adulteration in spices
- Detection of contamination and microbial examination techniques
For analytical methods concerning mycotoxins, pesticide residues, heavy metals, and microbiological analysis of spices, analysts should consult the appropriate FSSAI Manuals.
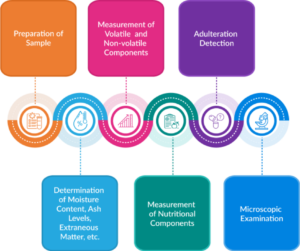
Fig. 2: Test Methods for Analysis of Foods – Spices and Condiments
Ethylene Oxide Testing: Addressing Emerging Concerns
FSSAI introduced a specific method for testing ethylene oxide in spices in 2023 after it was noted as a contaminant. This update is made to ensure that the levels of ethylene oxide in spices are within safe limits.
The method is selective and sensitive for analysing ethylene oxide residues in various matrices, including oilseeds, cereals, tea, spices, herbs, dehydrated fruits, food additives, and fresh produce. FSSAI outlines modified techniques for validation showing accurate and precise measurements across various food matrices.
Key points about ethylene oxide testing:
- The method uses gas chromatography-mass spectrometry (GC-MS)
- It can detect both ethylene oxide and its reaction product- 2-chloroethanol
Conclusion
The FSSAI’s comprehensive approach in regulating spices, condiments and related products, demonstrates India’s commitment to food safety and quality. These regulations ensure the authenticity and purity of spices and ensure safe consumption by determining and eradicating adulteration and contamination.
By serving as a regulatory body, FSSAI continues to uphold India’s reputation to provide a standard for premium high-quality spices.
References:
- Chapter 2.9 (Salt, Spices, Condiments and related products), Compendium on Food Safety and Standards (Food Products Standards and Food Additives) Regulation, 2011 (https://www.fssai.gov.in/upload/uploadfiles/files/Chapter%202_9%20(Salt%20Spices%2C%20Condiments%20and%20related%20products).pdf)
- APPENDIX B: Microbiological Requirements, Table: 3 Microbiological Standards for Spices and Herbs (https://www.fssai.gov.in/upload/uploadfiles/files/Appendix%20B.pdf)
- Guidance Document – FSSAI (https://www.fssai.gov.in/upload/uploadfiles/files/Guidance_Document_Spices_23_10_2018.pdf)
- Food Safety and Standards (Prohibition and Restriction of Sales) Regulation, 2011 (https://www.fssai.gov.in/upload/uploadfiles/files/Comp_Prohibition%20and%20Restrcition%20of%20sales%20X_03_04_2023.pdf)
- Maximum Residue Limits (MRLs) for spices and culinary Herbs (https://www.fssai.gov.in/upload/advisories/2024/04/6616351c775b5Order%20MRL%20Spices%20and%20culinary%20herbs.pdf)
- Food Safety and Standards (Contaminants, Toxins and Residues) Regulation, 2011 (https://www.fssai.gov.in/upload/uploadfiles/files/Compendium_Contaminants_Regulations_28_01_2022.pdf)
- Revised Manual on Spices, Herbs and Condiments size (https://fssai.gov.in/upload/uploadfiles/files/Manual_Revised_Spices_Herbs_22_06_2021.pdf)
- Residue Analysis of Ethylene Oxide and 2-Chloroethanol in Foods by Gas Chromatography Tandem Mass Spectrometry https://www.fssai.gov.in/upload/uploadfiles/files/Method%20for%20Residue%20analysis%20of%20Ethylene%20Oxide%20and%202%20Chloroethanol%20in%20Foods.pdf
Enquire Now
To enquire about our services please complete the form below and we will be in tough with you as soon as possible
Food Regulatory Services
- Consumer Product
- Compliance Services
- Licenses